
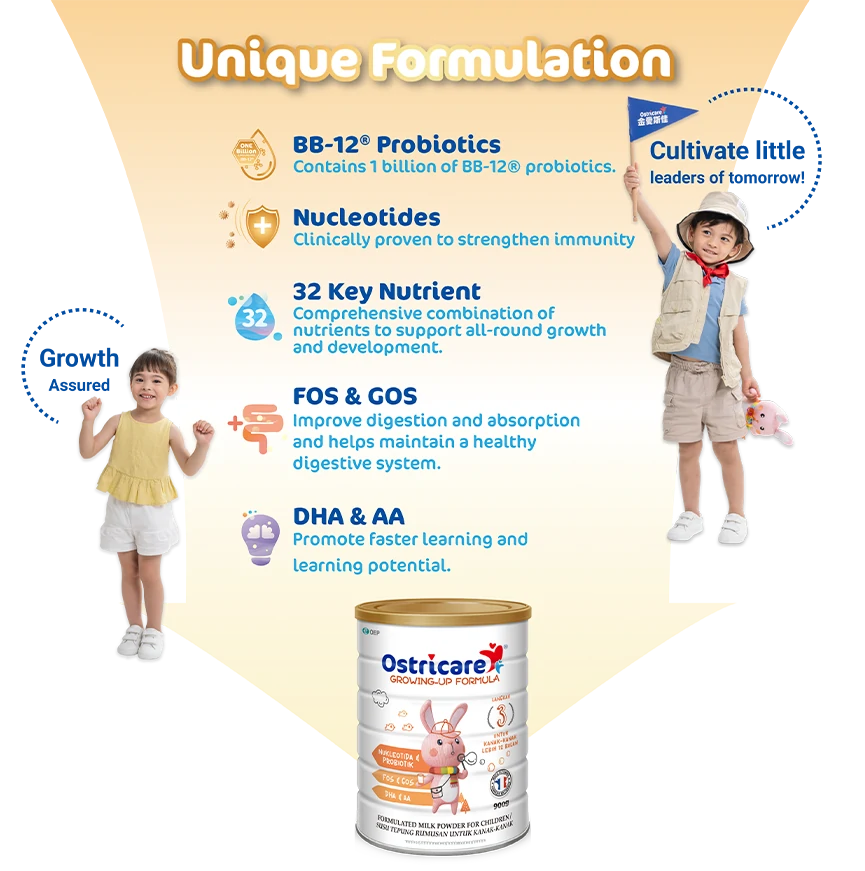
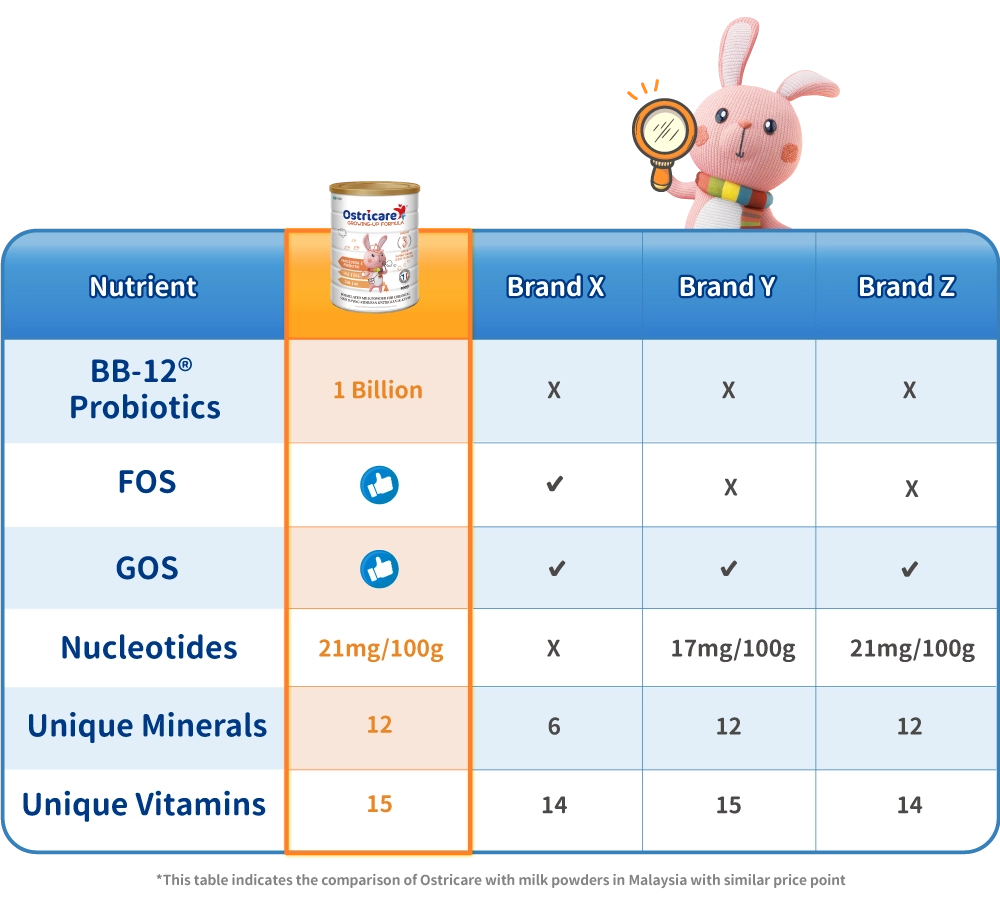
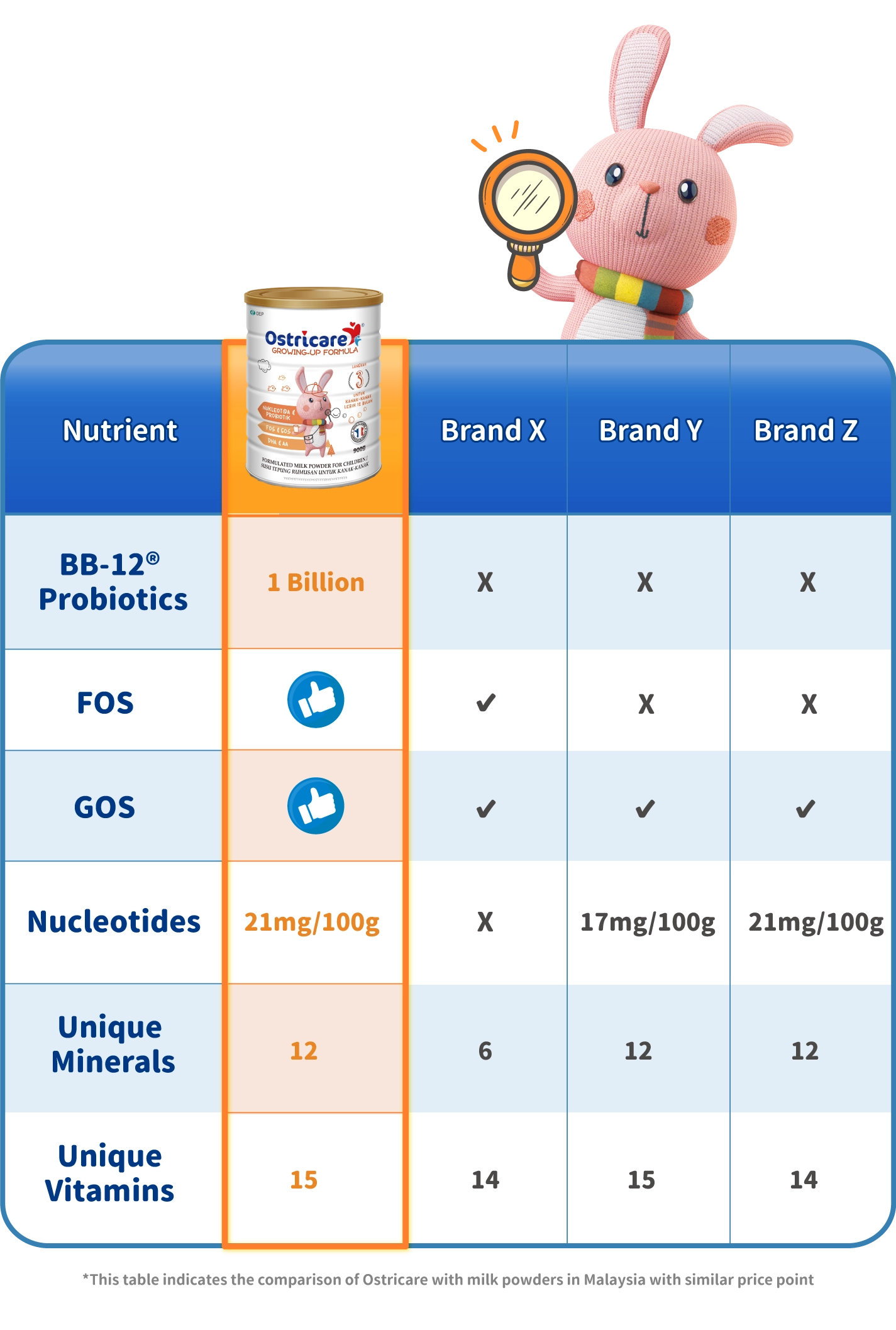
Ostricare milk powder is made from pure cow's milk from France. It offers two unique ingredients, nucleotides and BB-12® probiotics which are backed by clinical studies to reinforce children's innate defenses. Give your child all the protection, care and energy to explore the world without any worry!



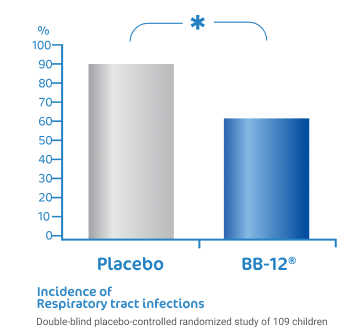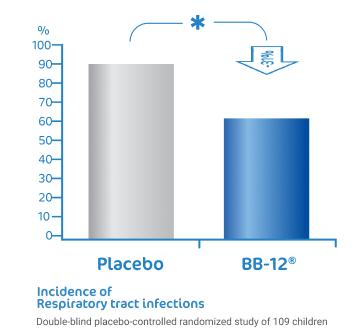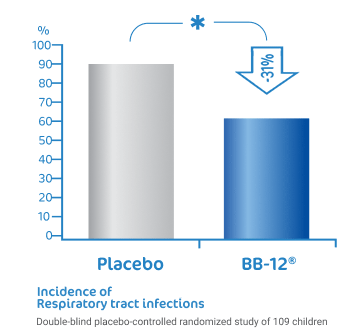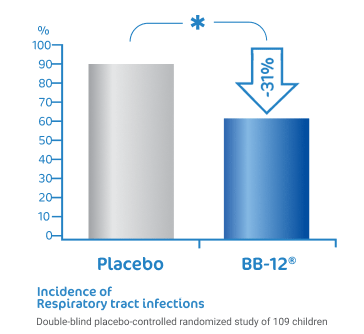
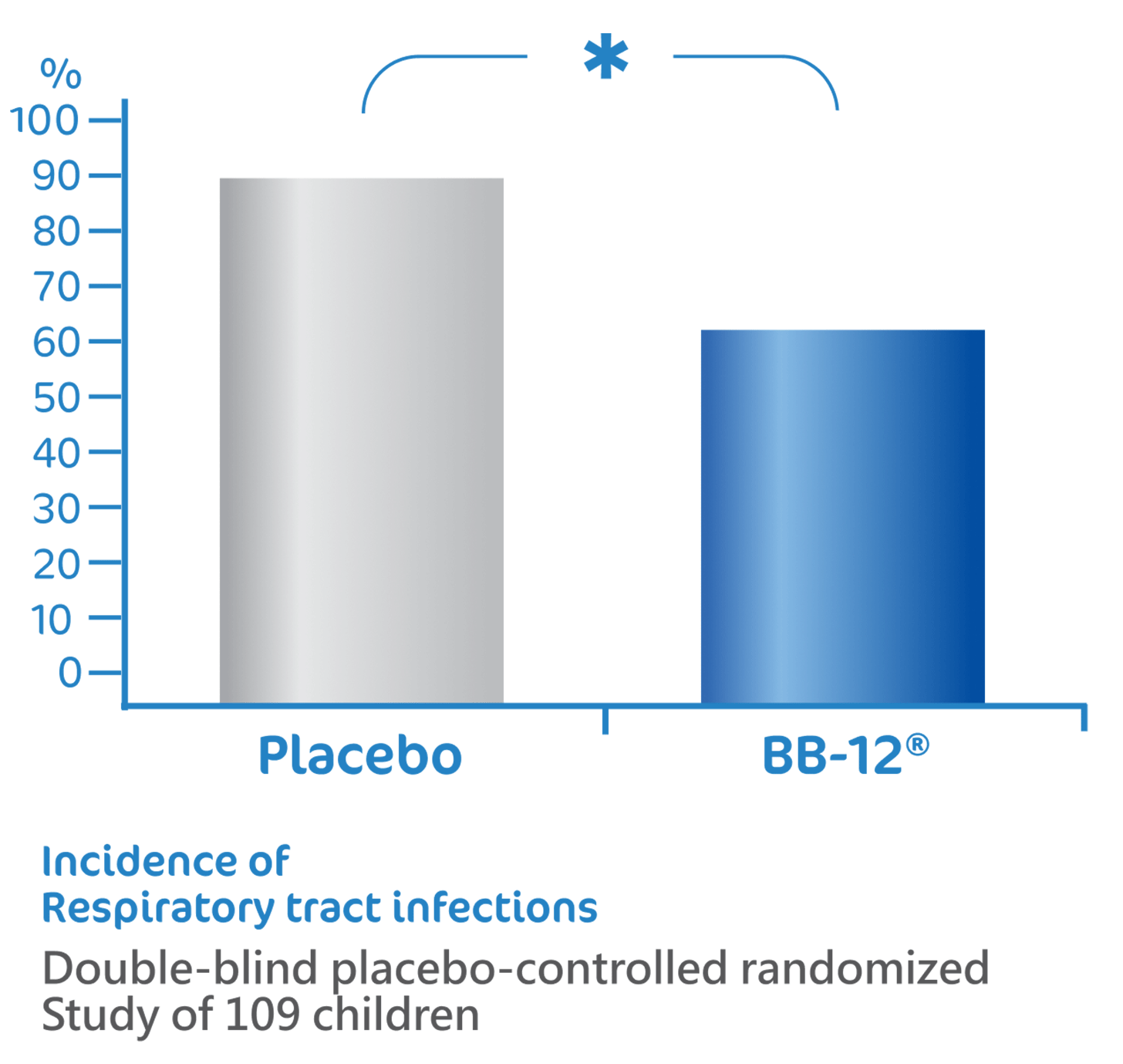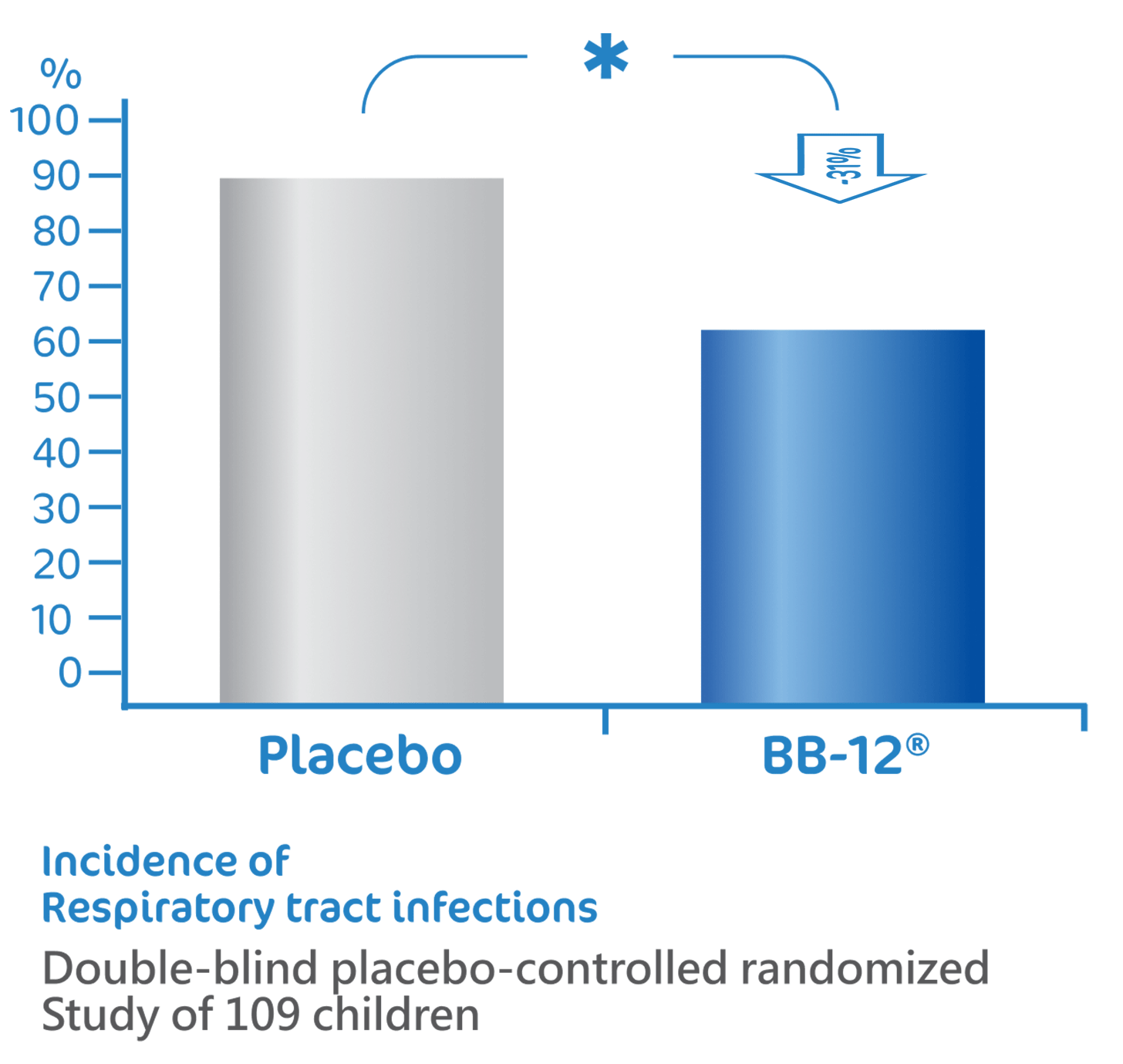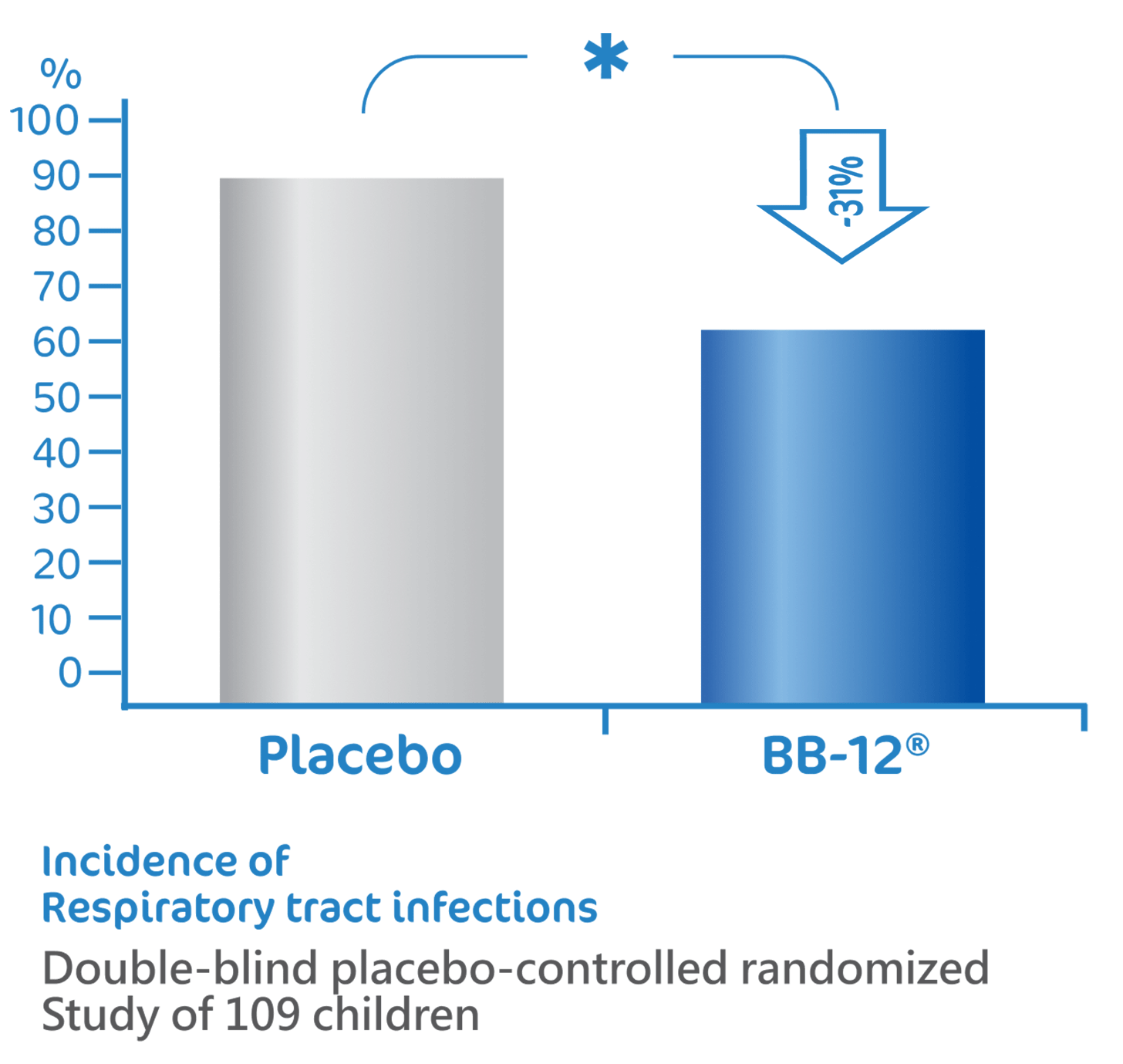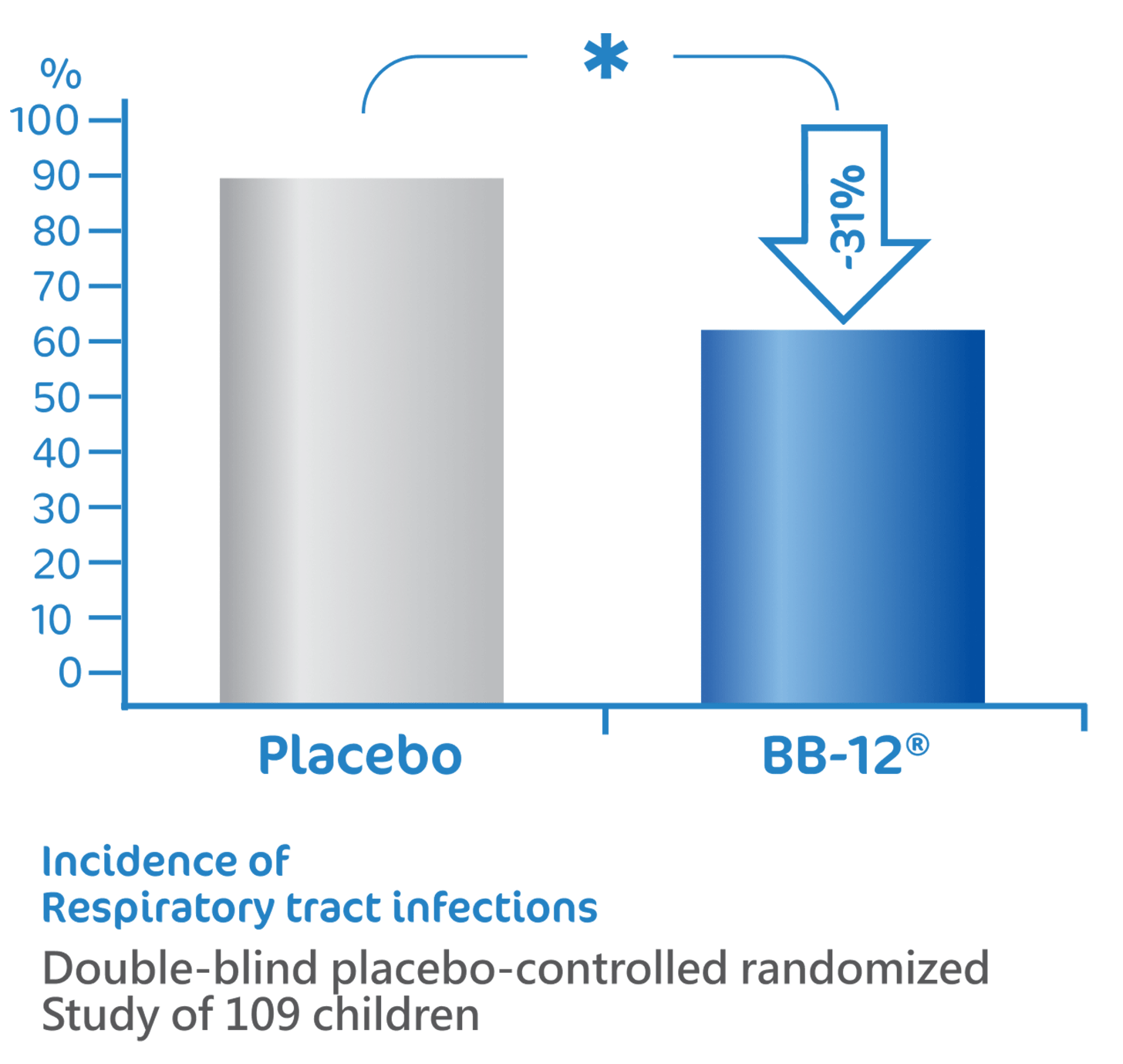
BB-12® reduce the risk of
respiratory tract infections1
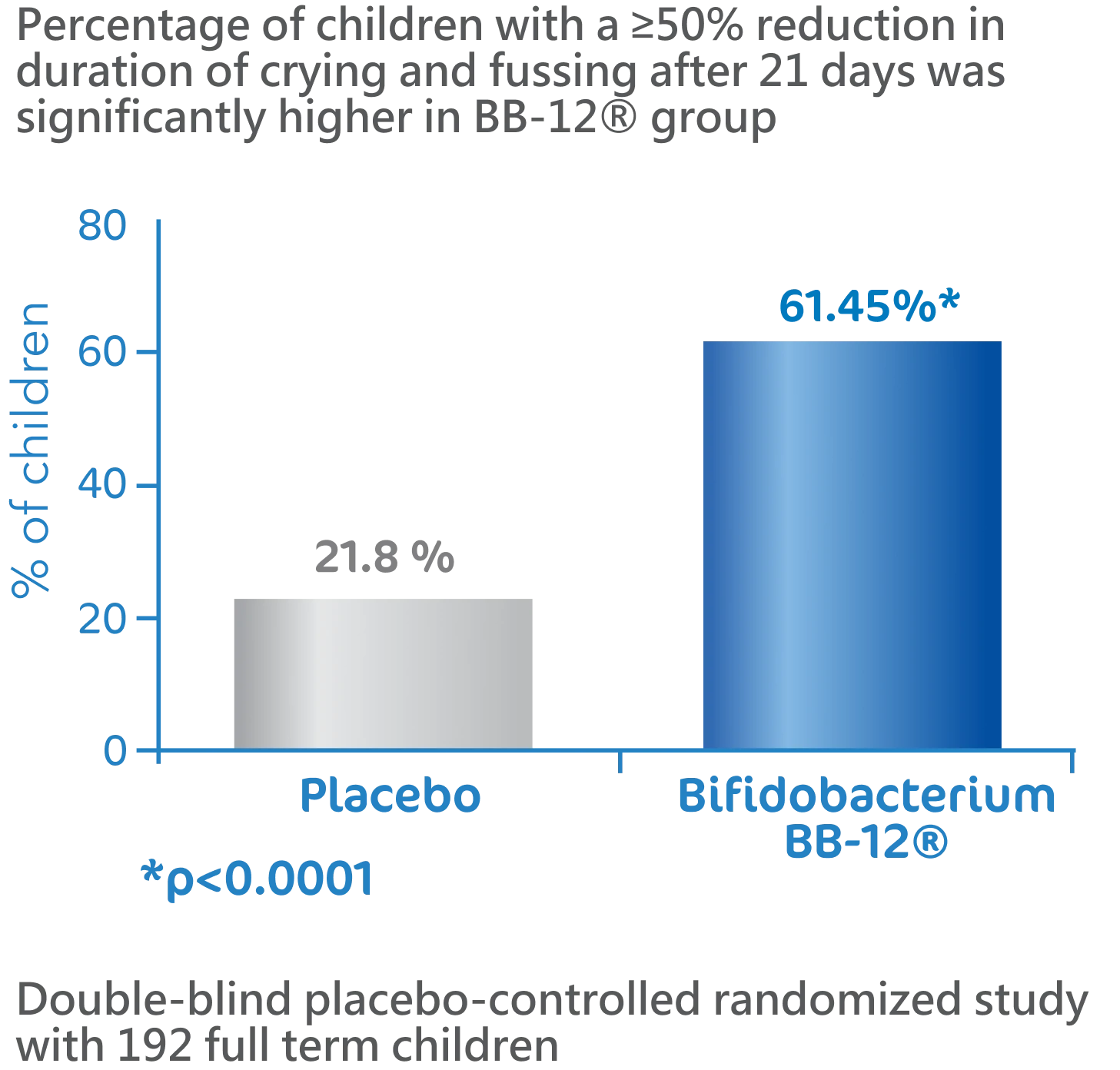
BB-12® is effective in reducing crying and fussing in children diagnosed with colic2
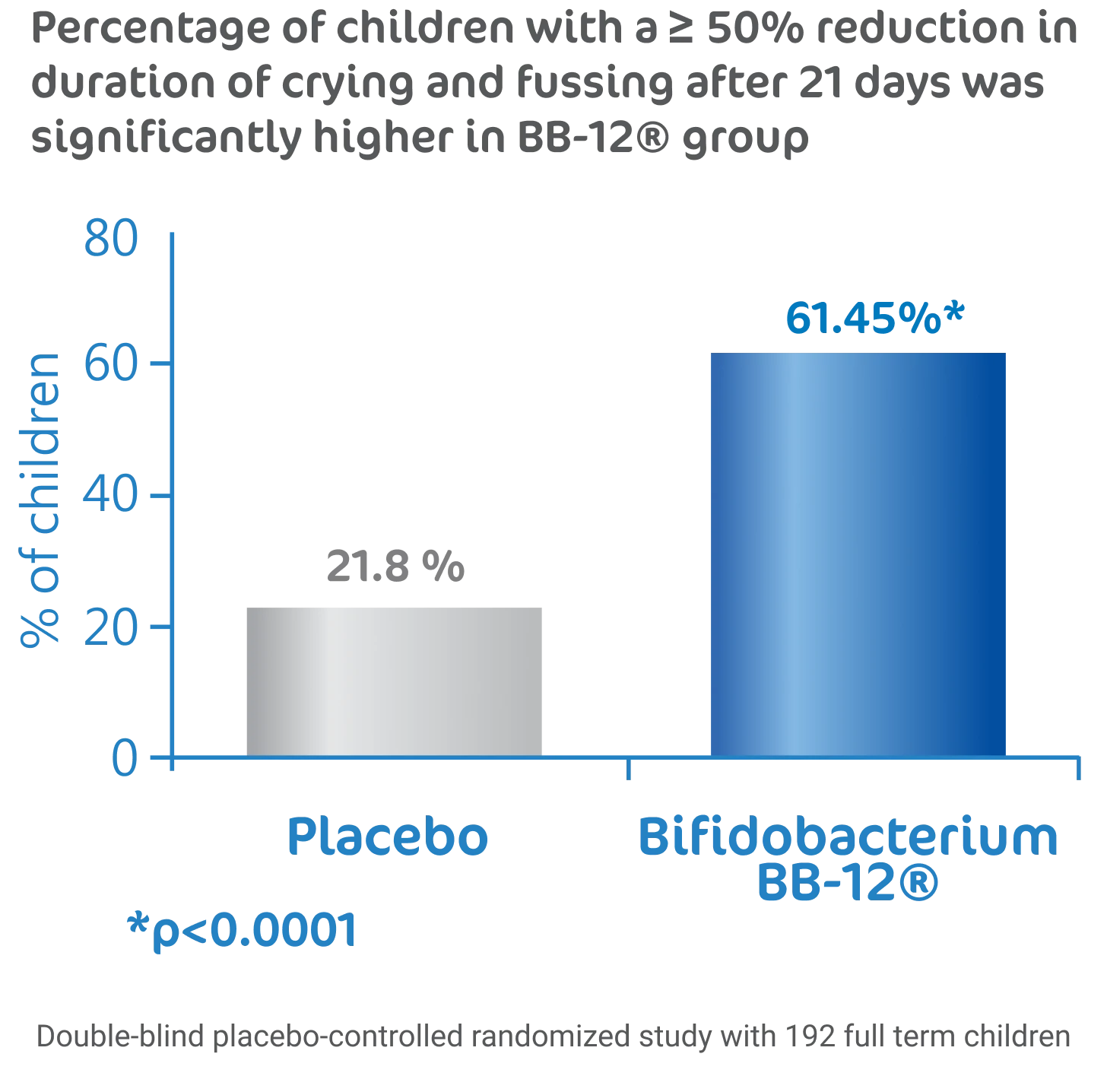
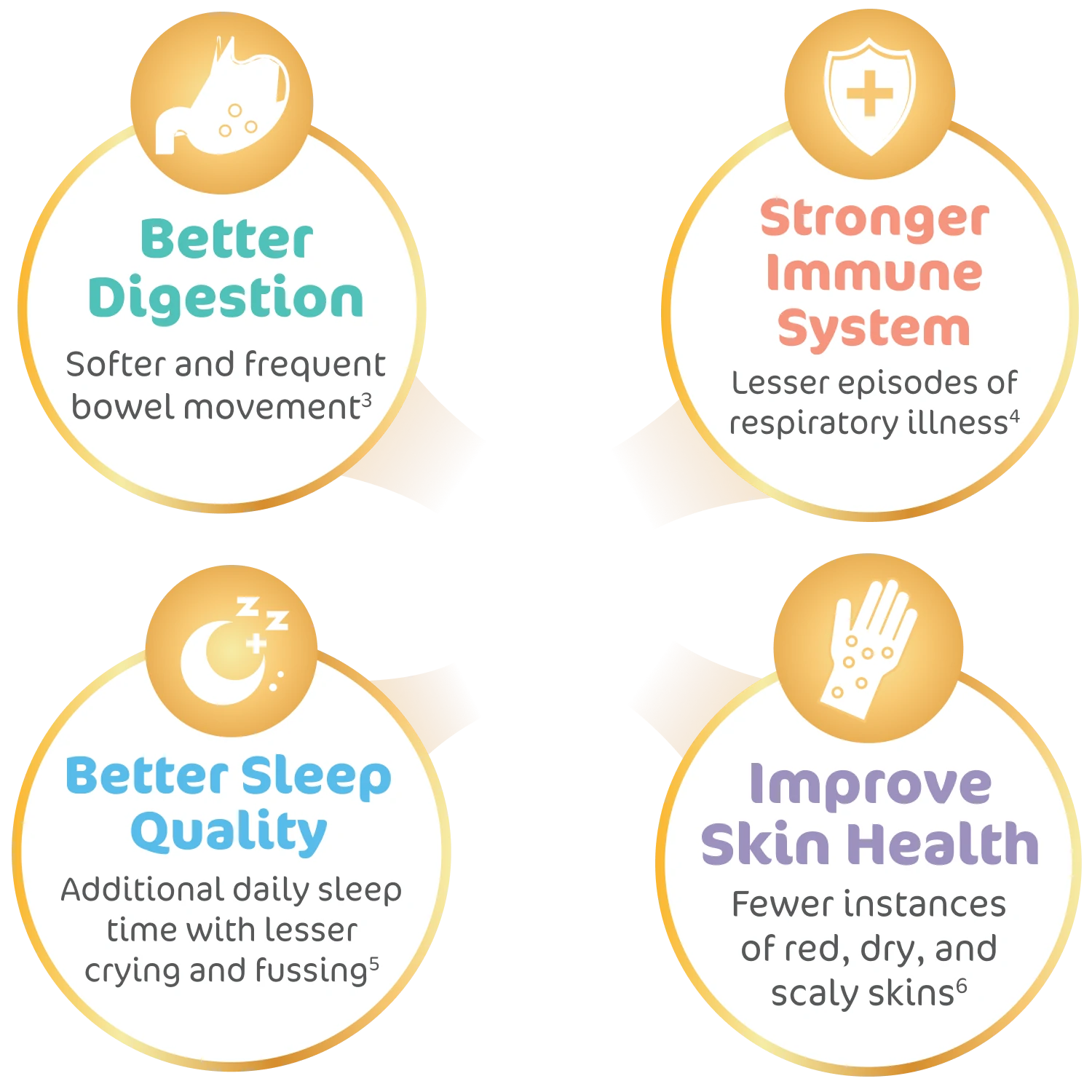
BB-12® probiotics is effective in boosting the natural immunity against respiratory tract infection and promoting better sleep quality for children.

The BB-12® strain from Chr. Hansen received a Generally Recognized As Safe (GRAS) status by
the Food and Drug Administration (FDA) in the US for both children and the general
population.8
In Europe, Bifidobacterium animalis has been granted Qualified
Presumption of Safety (QPS) status since 2007 by the European Food Safety Authority (EFSA) – a status
granted at species level.9








Resource:+
1.Jungersen et al. 2014. The Science behind the Probiotic Strain Bifidobacterium
animalis subsp. lactis
BB-12®. Microorganisms, 2(2), pp.92-110
2.Chen et al. 2021. Efficacy of Bifidobacterium animalis subsp. lactis, BB-12® on infant
colic–a
randomised, double-blinded, placebo-controlled study. Beneficial Microbes, 12(6), pp.531-540.
3.Vlieger et al. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination
with
Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: a randomised controlled
trial. British journal of nutrition, 102(6), pp.869-875.
4.Taipale et al., 2011. Bifidobacteriumanimalis subsp. lactis BB-12 in reducing the risk of
infections
in infancy. British Journal of Nutrition, 105(3), pp.409-416
5.Nocerino et al., 2020. The therapeutic efficacy of Bifidobacterium animalis subsp. lactis
BB‐12® in
infant colic: A randomised, double blind, placebo‐controlled trial. Alimentary pharmacology &
therapeutics, 51(1), pp.110-120.
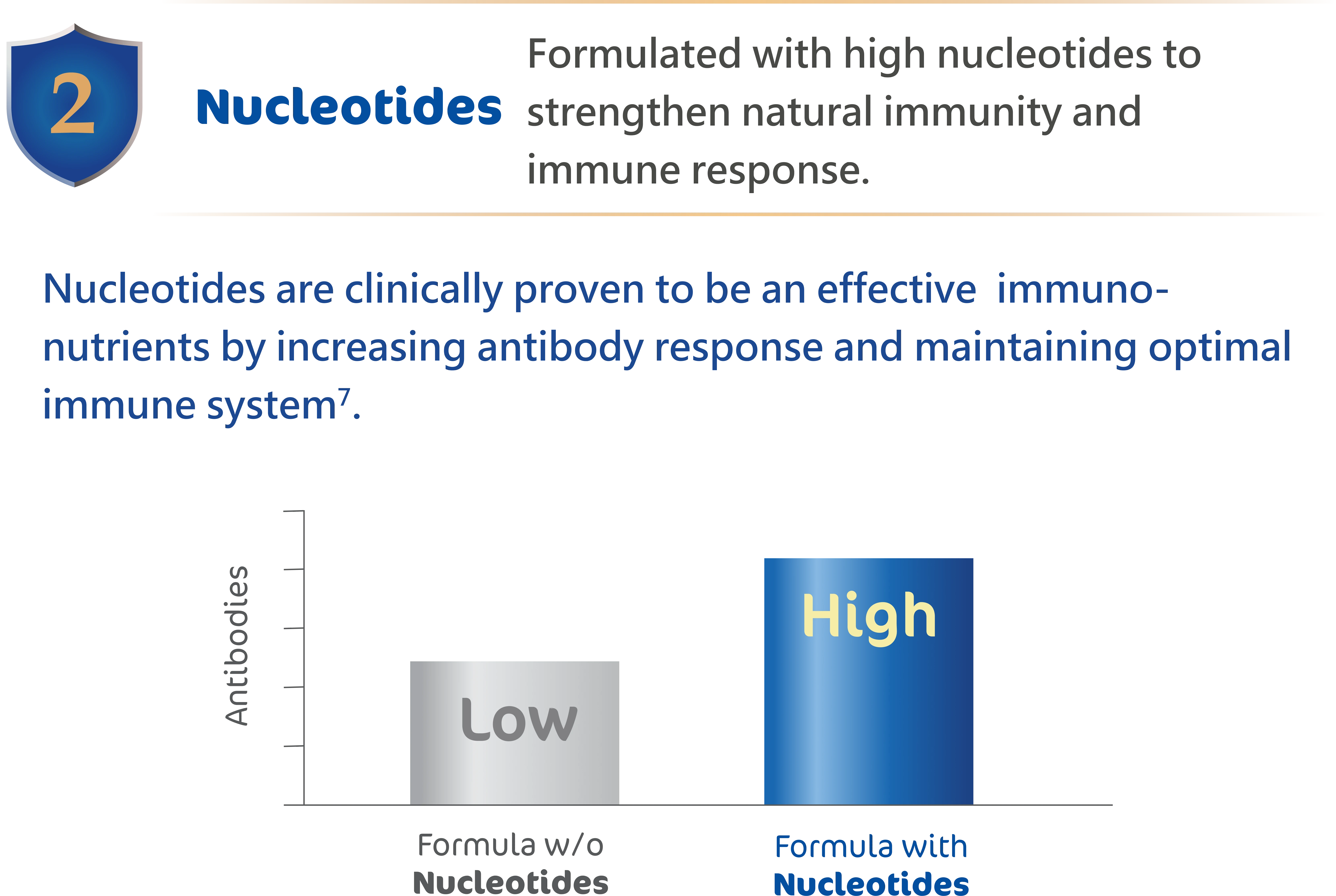
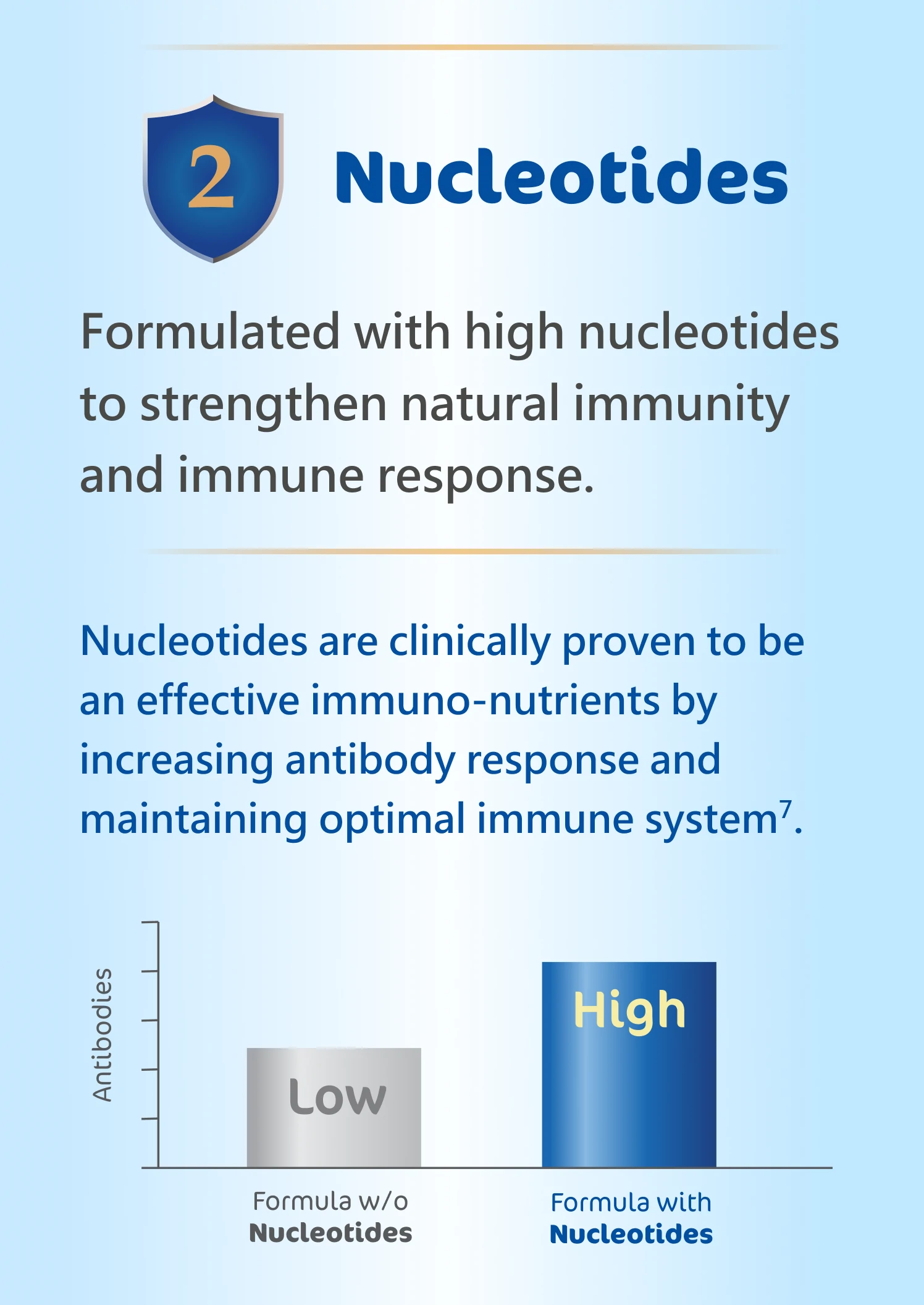
6.Isolauri et al., 2021. Nucleotides as optimal candidates for essential nutrients in living
organisms:
A review. Journal of Functional Foods, 82, p.104498.
7.Yau et al., 2003. Effect of nucleotides on diarrhea and immune responses in healthy term
infants in
Taiwan. Journal of pediatric gastroenterology and nutrition, 36(1), pp.37-43.
8.Food and Drug Administration. GRAS Notice Inventory > Agency Response Letter GRAS Notice No
GRN
000049. 2002.
9.EFSA Panel on Biological Hazards (BIOHAZ). Statement on the update of the list of
QPS-recommended
biological agents intentionally added to food or feed as notified to EFSA 3: Suitability of taxonomic
units notified to EFSA until September 2015. EFSA Journal. 2015;13:4331.




BB-12® reduce the risk of
respiratory tract infections1
BB-12® is effective in reducing crying and fussing in children diagnosed with colic2


The BB-12® strain from Chr. Hansen received a Generally Recognized As Safe (GRAS) status by
the Food and Drug Administration (FDA) in the US for both children and the general
population.8
In Europe, Bifidobacterium animalis has been granted Qualified
Presumption of Safety (QPS) status since 2007 by the European Food Safety Authority (EFSA) – a status
granted at species level.9









Resource:+
1.Jungersen et al. 2014. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis
BB-12®. Microorganisms, 2(2), pp.92-110
2.Chen et al. 2021. Efficacy of Bifidobacterium animalis subsp. lactis, BB-12® on infant
colic–a
randomised, double-blinded, placebo-controlled study. Beneficial Microbes, 12(6), pp.531-540.
3.Vlieger et al. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination
with
Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: a randomised controlled
trial. British journal of nutrition, 102(6), pp.869-875.
4.Taipale et al., 2011. Bifidobacteriumanimalis subsp. lactis BB-12 in reducing the risk of
infections
in infancy. British Journal of Nutrition, 105(3), pp.409-416
5.Nocerino et al., 2020. The therapeutic efficacy of Bifidobacterium animalis subsp. lactis
BB‐12® in
infant colic: A randomised, double blind, placebo‐controlled trial. Alimentary pharmacology &
therapeutics, 51(1), pp.110-120.
6.Isolauri et al., 2021. Nucleotides as optimal candidates for essential nutrients in living
organisms:
A review. Journal of Functional Foods, 82, p.104498.
7.Yau et al., 2003. Effect of nucleotides on diarrhea and immune responses in healthy term
infants in
Taiwan. Journal of pediatric gastroenterology and nutrition, 36(1), pp.37-43.
8.Food and Drug Administration. GRAS Notice Inventory > Agency Response Letter GRAS Notice No
GRN
000049. 2002.
9.EFSA Panel on Biological Hazards (BIOHAZ). Statement on the update of the list of
QPS-recommended
biological agents intentionally added to food or feed as notified to EFSA 3: Suitability of taxonomic
units notified to EFSA until September 2015. EFSA Journal. 2015;13:4331.




